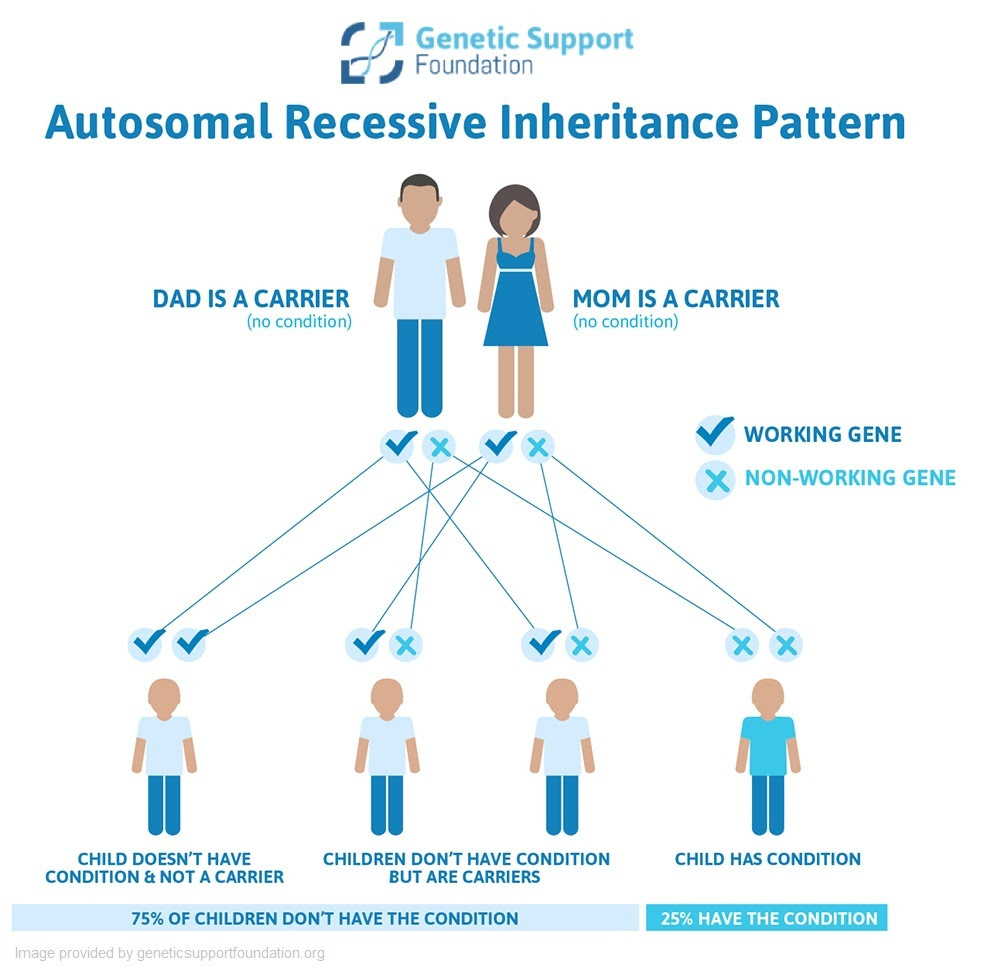
My colleagues and I were recently discussing some issues around carrier screening, particularly when it comes to variants of uncertain significance (VUS). As the name implies, VUSs are changes that are found in a gene that have not been seen enough to know whether they affect how the gene works (and thus can cause disease) or whether they are benign changes in the gene that do not affect how the gene works (and thus do not cause disease). The vast majority of genetic conditions on carrier screening panels are autosomal recessive, although some may be X-linked.
Labs who perform carrier screening tests have traditionally not reported out VUS findings. Part of the rationale for this is that carrier screening is not meant to identify or diagnose a genetic condition in a patient; it is designed to determine someone’s chances to have a child with a genetic condition. Those are two very different scenarios, and having uncertain genetic test results can impact patients in these circumstances quite differently.
It was my (and my colleagues’) understanding that VUS findings are not reported or tracked on the laboratory back end with genetic carrier screening. We recently had a situation where we received an updated carrier screening report for a patient. The patient initially had expanded carrier screening during the first trimester of her pregnancy that was all ‘negative’. As the patient was approaching her due date and was in the final weeks of her pregnancy, the lab issued an amended report stating that there was previously a VUS identified on this patient’s testing, and that this variant has now been updated to “likely pathogenic”; this means she is now a carrier for a serious childhood-onset condition. No VUS was identified on the patient’s initial report, and it was not clearly communicated that the lab would be tracking and potentially updating the results.
Typically, if someone is found to be a carrier for a recessive genetic condition, testing would be offered to their partner. If the partner is also a carrier for the same condition, there is a 25% chance that the pregnancy would be affected. The option for invasive diagnostic testing, such as amniocentesis, can then be offered to patients who would like to know with certainty if the pregnancy will be affected. Some people choose to do diagnostic testing because they would like to have the opportunity to gather information and prepare themselves, and some people may change their plans for a pregnancy based on the test results. Identifying carrier status this late in a pregnancy significantly limits those options for many people.
This situation brought up a slew of follow-up questions. Do other labs track VUS findings on carrier screening? If a patient had carrier screening 5 years ago and the lab that did their testing is not updating the results with new findings, should individuals have repeat carrier screening? If so, after how long? Should labs be reporting or keeping track of VUS findings? How much do we as providers need to emphasize the potential for VUS findings on carrier screening (particularly since most labs do not track or report them)?
On top of the onslaught of unanswered questions that followed was the stress and anxiety on the part of the patient but also on the provider who had to relay this information to the patient. Updates such as this have the potential to emotionally and mentally complicate the remainder of the pregnancy for individuals in this circumstance, particularly if they are unexpected. Patients with these surprising updates may end up spending the last couple weeks in the pregnancy worried about whether or not their baby will have a significant and potentially life-limiting medical condition, which is not what anyone wants the end of their pregnancy to look like.
What do you think? Should labs track/update/report VUS findings on genetic carrier screening? Sound off in the comments below!
Related Content: Carrier Screening 101